Chemical Reactions and Equations Class 10
Important Questions Science Chapter 1
1. Which of the following is a displacement reaction?

Explaination: Reason: Here sodium (Na) displaces to form sodium hydroxide.
(a) Fe (III) chloride and water
(b) Fe (II) chloride and water
(c) Fe (II) chloride and hydrogen gas
(d) Fe (III) chloride and hydrogen gas
Explaination: Reason: 2Fe + 6HCl → 2FeCl3 (Iron (III) chloride) + 3H2
What is the method of balancing chemical equation?
Solution:
Hit and trial method is used for balancing simple chemical equations. In this method, coefficients before the symbols/formulae of the reactants and products are adjusted in such a way that the total number of atoms of each element on both the sides becomes equal.
What are the different ways can make more informative about the chemical equation?
Solution:
1. By indicating the “physical states” of the reactants and products.
2. By indicating the “heat changes” taking place in the reaction.
3. By indicating the “conditions” under which the reaction takes place.
Give balanced equations, wherever possible, or where this is not possible, explain the following by means of examples:
i) A reaction which gives out heat.
ii) A reaction which takes place with the help of sunlight.
iii) A reaction which is brought about by electric current.
iv) A reversible reactions.
(v) A reaction with a solid and gas which produces heat.
Balance the following chemical equations:

Suggest two ways to check the rancidity of food articles.
Answer:
(a) Keep the articles in airtight containers,
(b) Keep the articles in refrigerator.
Name two metals which donot get corroded.
Answer:
Gold (Au) and platinum (Pt) do not get corroded.
Select (i) combination reactions (ii) decomposition reactions and displacement reactions from the following
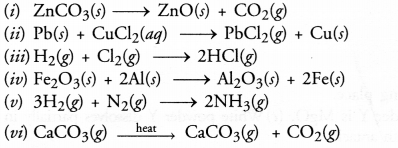
Answer:
(i) Decomposition reaction
(ii) Displacement reaction
(iii) Combination reaction
(iv) Displacement reaction
(v) Combination reaction
(vi) Decomposition reaction.
Observe the given figure and answer the following questions.
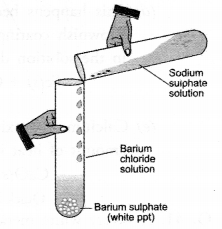
(a) Write the complete balanced reaction for the reaction that takes place.
(b) Type of reaction involved.
(c) Is there any precipitate formed.
(d) If any precipitate formed, write the colour of the precipitate.
Answer:
(b) It is a double displacement reaction
(c) Yes, a precipitate of barium sulphate is formed.
(d) The precipitate is white in colour.
Question 11.
You are given the following materials :
(i) Iron nails
(ii) Copper sulphate solution
(iii) Barium chloride solution
(iv) Copper powder
(v) Ferrous sulphate crystals
(vi) Quick lime.
Identify the type of chemical reaction taking place when :
(a) Barium chloride solution is mixed with copper sulphate solution and a white precipitate is observed.
(b) On heating, copper powder in air in a china dish, the surface of copper powder becomes black.
(c) On heating green ferrous sulphate crystals, reddish brown solid is left and a gas having smell of burning sulphur is noticed.
(d) Iron nails when left dipped in blue copper sulphate solution become brownish in colour and blue colour of copper sulphate solution fades away.
(e) Quick lime reacts vigorously with water releasing a large amount of heat.
Answer:
(a) The reaction is double displacement in nature. ‘![]()
(b) It is an example of combination reaction.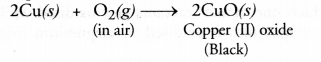
(c) The green crystals of ferrous sulphate have the chemical formula FeS04.7H20. Upon heating, they lose molecules of water of crystallisation.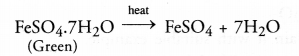
Upon further heating, ferrous sulphate undergoes decomposition reaction as follows :
Both the gases evolved have the smell of burning sulphur.
(d) This happens because of displacement reaction. Iron displaces copper form copper sulphate solution. Brownish coating of copper gets deposited on the iron nails. As the concentration of copper sulphate in the solution decreases, the blue colour of the solution slowly fades.![]()
(e) Calcium hydroxide is formed as a result of combination reaction. It is highly exothermic. A large amount of heat is evolved accompanied by hissing sound.![]()
Question 12.
A house wife wanted her house to be white washed. She bought 10 kg of quick lime from the market and dissolved in 30 litres of water.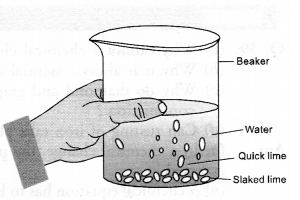
Answer:
She noticed that water started boiling even when it was not being heated. Give reason for her observation. Write the corresponding equation and name the product formed.
A suspension of slaked lime also called calcium hydroxide is formed when water is added to quick lime.![]()
Since the reaction is highly exothermic, the solution started boiling although it was not being heated. The suspension of slaked lime is allowed to cool for sometime, preferably overnight. It is then decanted and the liquid obtained is used for white washing.
Question 13.
What is a redox reaction ? When a magnesium ribon burns in air with a dazzling flame and forms a white ash; is magnesium oxidised or reduced ? Why ?
Answer:
A redox reaction is a chemical reaction in which one of the reactants gets oxidised while the other is reduced simultaneously. In the reaction under study, magnesium is oxidised to magnesium oxide since the metal has gained oxygen.
Question 14.
Give one example each of :
(i) Thermal decomposition reaction
(ii) Electrolytic decomposition reaction
(iii) Photo decomposition reaction. (CBSE 2014)
Answer: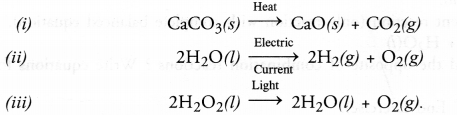
Question 15.
Aluminium is a reactive metal but is still used for packing food articles. Why ?
Answer:
From the position of the aluminium (Al) metal in the activity series, it seems to be quite reactive. However, it is not so reactive. Actually, when the metal is kept in air or oxygen for sometime, it is converted into its oxide called aluminum oxide (Al2O3). This gets deposited as the surface of the metal as a thin coating. It is rather passive which means that it is not reactive. Therefore, the metal is used for packing food articles which do not get spoiled under the foil.
Question 16.
Name two salts that are used in black and white photography.
Answer:
Both silver chloride and silver bromide are used in black and white photography
.Question 17.
Give an example of a double displacement reaction (only reaction with complete balanced equation).Answer:
HCl(aq) + NaOH(g) ——–> NaCl(aq) + H2O(l)
What happens chemically when quick lime is added to water ?
Answer:
Calcium hydroxide (or slaked lime) is formed accompanied by a hissing sound. So much heat is evolved during the reaction that the reaction mixture starts boiling. The chemical equation for the reaction is :
Identify the compound which is oxidised in the following reaction
H2S + Br2 ———–> 2HBr + S.
Answer:
H2S is oxidised to S because H2S has lost hydrogen
(a) Why is combustion reaction an oxidation reaction ?
(b) How will you test whether the gas evolved in a reaction is hydrogen ?
(c) Why does not silver evolve hydrogen on reacting with dillute sulphuric acid ?
Answer:
(a) Combustion reaction is an oxidation reaction because it is always carried in the presence of air or oxygen. For example,
CH4(s) + 2O2(g) ——–> CO2(g) + 2H2O (l)
(b) Bring a burning match stick close to the mouth of the tube from which hydrogen gas escapes. The gas will immediately catch fire and this will be accompanied by pop sound.
(c) Silver is a less reactive metal in the sense that it occupies a place below hydrogen in the reactivity series. Therefore it does not evolve hydrogen gas on reacting with either dilute sulphuric acid or dilute hydrochloric acid.


Comments
Post a Comment